Serious complications and risk of re-operation after Dupuytren’s disease surgery: a population-based cohort study of 121,488 patients in England
Study design and setting
We conducted a longitudinal nationwide population-based cohort study and self-controlled case series using Hospital Episode Statistics (HES) for National Health Service (NHS) England linked with the Office for National Statistics (ONS) mortality data.
Participants
Our study population consisted of any surgical procedure for Dupuytren’s disease undertaken between 1 April 2007 and 31 March 2017 in a patient over 18 years of age. We extracted information from the Admitted Patient Care (APC) data in the HES dataset. Operations with missing side code and operations that had less than 90 days follow-up after surgery (total n = 7286; 4.6%) were excluded from the analysis of local complications. For systemic complications, only operations with less than 90 days follow-up after surgery (n = 4743; 3%) were excluded from the analysis. Operations with missing laterality code (n = 2577; 1.63%) were excluded from the analysis of re-operation rate.
Exposure
The exposure was DD surgical procedures. These operations were grouped into percutaneous needle fasciotomy (PNF), limited fasciectomy (LF) and dermofasciectomy (DF). Surgeries were identified using a pre-specified list of the International Classification of Diseases, 10th revision (ICD-10) codes and the 4th edition of the Office of Population Census and Survey (OPCS-4) codes (Supplementary Table 1).
Outcomes
The outcomes were any serious local or systemic adverse events, which were identified using ICD-10 and OPCS-4 codes (see Supplementary Table 2a,b). Serious local complications were defined as surgical site infection (SSI) requiring wound debridement, wound dehiscence requiring surgical closure, neurovascular and tendon injury requiring surgical repair, and finger amputation that occurred in the operated hand. SSI and wound dehiscence were also combined into one category “wound complications”. Serious systemic complications studied were stroke, lower respiratory tract infection (LRTI), acute myocardial infarction (AMI), pulmonary embolism (PE), urinary tract infection (UTI), acute kidney injury (AKI), and death.
Re-operation after DD surgery was a secondary outcome. Within OPCS, revision procedure codes are only available for revision LF and revision DF. Because finger specific codes are not available in OPCS, someone having a second operation on the same hand that was coded as a primary procedure in OPCS might be undergoing true primary surgery on a different finger, or true revision surgery following a primary procedure. This limitation of the OPCS coding system prevents analysis of the number of procedures per finger, and therefore we used the term ‘re-operation’ to describe any further surgery on the same hand following primary surgery.
Data source
We analysed a bespoke extract from prospectively collected APC data in the HES dataset. The HES APC data extract contained all incident and prevalent episodes of admitted care for all adult patients at all National Health Services (NHS) hospitals, including NHS treatment provided in the independent sector, in England with at least one DD treatment episode between 1st April 2007 and 31st March 2017. This dataset was linked with the ONS mortality data for the purpose of survival analysis in this study.
Study size
The final HES APC extract included 158,119 procedures performed in 121,488 patients.
Statistical methods
Cumulative incidence of postoperative complications
Cumulative (unadjusted) incidence was calculated, and 95% confidence intervals (CI) were estimated using the Rothman and Greenland method9, for serious local and systemic complications within 30 and 90 days after all DD surgery. We selected 30 and 90 days after surgery as recommended by the NHS Outcome Framework guideline10. Only complications severe enough to require re-admission to hospital were included, as these were coded in HES APC.
Self-controlled case series
We used a self-controlled case series (SCCS) study design (Fig. 1) to estimate the relative risk of serious systemic complications in the first 30 and 90 days following surgery. This design is a within-case comparison, requiring no separate comparator: each case acts as its own control to eliminate any time constant confounders11,12. We further hypothesised that procedures generally performed under general or regional anaesthesia (LF or DF) would increase the risk of serious systemic complications more than those routinely carried out under local anaesthesia (PNF). Our analysis was limited to the first primary DD surgery as studying beyond the first primary DD surgery could result in an overlap between an exposure period of one surgery and a baseline period of another surgery.
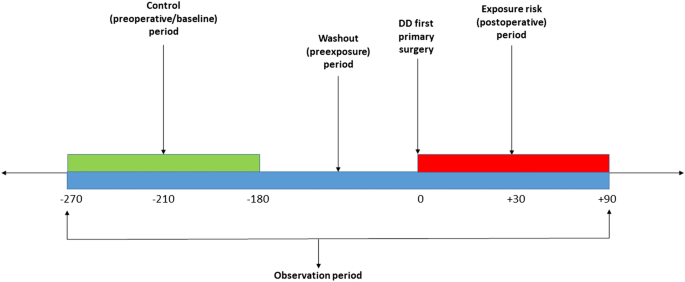
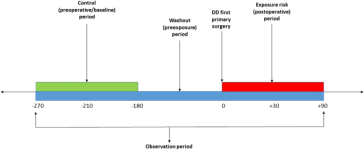
Schematic representation of the self-controlled Case Series (SCCS) model showing exposure risk period (red), control or baseline period (green), washout period and observation periods (dark blue).
The baseline observation period was defined, for 90-day adverse events, as any time between 270 and 180 days before surgery (Fig. 1), and for 30-day events from 210 to 180 days before surgery. The rate of serious systemic events that occurred within 30 or 90 days after surgery, the exposure risk period, were then compared with their rate in each corresponding baseline risk period. A washout (pre-exposure) period including the 6 months before surgery was used to comply with one of the key assumptions of the SCCS, that the occurrence of an adverse event should not affect the probability of exposure13. In this case, the occurrence of any serious systemic adverse event would temporarily decrease the probability of having DD surgery.
Less than 1% of patients in our cohort died after surgery prior to experiencing any systemic adverse event, so we did not account for death in our analysis. As age is considered a time-varying confounder, it was adjusted for by including it in the SCCS model. Age was categorised into bands in order for the underlying Poisson model to accommodate rare events. Age categories for most complications were 40–59 years, 60–64, 65–69, 70–74, 75–79, 80–84, 85–89, 90–94 and 95 and above. For two complications, AKI and PE, different age categories were used due to a lower incidence of complications in younger and older patients, and hence a lack of model convergence.
Conditional Poisson regression offset by the natural logarithm of risk intervals was used to study the association between exposure to surgery and the occurrence of serious adverse events within 30 or 90 days after surgery. The incidence rate ratio (IRR) and 95% CI were calculated for each adverse event. Interactions with gender were examined by adding this variable to the SCCS model as a multiplicative interaction. The analysis was also repeated with the case cohorts stratified by type of surgery.
Survival analysis
Kaplan Meier survival estimates were used to determine the cumulative probability of re-operation after primary surgery. The analysis was limited to the first re-operation following all primary DD surgery, and for each surgical subtype. Multivariable Cox proportional hazards regression analysis was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for each potential determinant of re-operation. Age, gender, grouped Charlson comorbidity index, and IMD decile were added to the Cox regression models as determinants. Operations were followed up from the date of primary DD operation until first re-operation on the same hand, death, loss-to follow-up, or end of the study period.
Except for ethnicity, missing data were less than 1% and considered to be ‘missing completely at random’. Therefore, records with missing data were excluded from the analysis and we utilised a complete cases analysis approach. Statistical analyses were performed using Stata MP (StataCorp. 2017. Stata Statistical Software: v15. College Station, TX). Any absolute value between 0 and 5 was suppressed at the reporting stage to comply with the HES and ONS disclosure policies14.
Patient and public involvement
This study addresses one of the top 10 priorities for clinical research in hand and wrist surgery, as determined by the James Lind Alliance and British Society for Surgery of the Hand Priority Setting Partnership8.
Ethical approval
The study was approved by the University of Oxford Research Services (project ID 12787), and the NHS Data Access Advisory Group. Ethical approval was not required. It was conducted in accordance with the NHS Digital data sharing agreement (DARS-NIC-29827-Q8Z7Q) and registered at ClinicalTrials.gov (NCT03573765).



