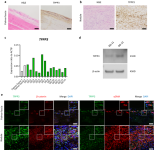
C-X-C domain ligand 14-mediated stromal cell–macrophage interaction as a therapeutic target for hand dermal fibrosis
Shih, B. & Bayat, A. Scientific understanding and clinical management of Dupuytren disease. Nat. Rev. Rheumatol. 6, 715–726 (2010).
Lanting, R., Broekstra, D. C., Werker, P. M. N. & van den Heuvel, E. R. A systematic review and meta-analysis on the prevalence of Dupuytren disease in the general population of Western countries. Plast. Reconstr. Surg. 133, 593–603 (2014).
Carr, L., Michelotti, B., Brgoch, M., Hauck, R. & Ingraham, J. Dupuytren disease management trends: A survey of hand surgeons. Hand. (N. Y). 15, 97–102 (2020).
Bainbridge, C. et al. Current trends in the surgical management of Dupuytren’s disease in Europe: an analysis of patient charts. Eur. Orthop. Traumatol. 3, 31–41 (2012).
Musumeci, M. et al. Dupuytren’s disease therapy: targeting the vicious cycle of myofibroblasts? Expert Opin. Ther. Targets. 19, 1677–1687 (2015).
Dolmans, G. H. et al. Wnt signaling and Dupuytren’s disease. N. Engl. J. Med. 365, 307–317 (2011).
Ng, M. et al. A genome-wide association study of Dupuytren disease reveals 17 additional variants implicated in fibrosis. Am. J. Hum. Genet. 101, 417–427 (2017).
Varallo, V. M. et al. Beta-catenin expression in Dupuytren’s disease: potential role for cell-matrix interactions in modulating beta-catenin levels in vivo and in vitro. Oncogene. 22, 3680–3684 (2003).
Ten Dam, E. J., van Beuge, M. M., Bank, R. A. & Werker, P. M. Further evidence of the involvement of the Wnt signaling pathway in Dupuytren’s disease. J. Cell Commun. Signal. 10, 33–40 (2016).
van Beuge, M. M., Ten Dam, E. J., Werker, P. M. & Bank, R. A. Wnt pathway in Dupuytren disease: connecting profibrotic signals. Transl. Res. 166, 762–771.e3 (2015).
Samulėnas, G. et al. Evaluation of WNT signaling pathway gene variants. Genes (Basel). 12, 1293 (2021).
Izadi, D. et al. Identification of TNFR2 and IL-33 as therapeutic targets in localized fibrosis. Sci. Adv. 5, eaay0370 (2019).
Verjee, L. S. et al. Unraveling the signaling pathways promoting fibrosis in Dupuytren’s disease reveals TNF as a therapeutic target. Proc. Natl. Acad. Sci. USA. 110, E928–E937 (2013).
Akbar, M. et al. Attenuation of Dupuytren’s fibrosis via targeting of the STAT1 modulated IL-13Rα1 response. Sci. Adv. 6, eaaz8272 (2020).
Layton, T. B. et al. A vasculature niche orchestrates stromal cell phenotype through PDGF signaling: Importance in human fibrotic disease. Proc. Natl. Acad. Sci. USA. 119, e2120336119 (2022).
Staverosky, J. A., Pryce, B. A., Watson, S. S. & Schweitzer, R. Tubulin polymerization-promoting protein family member 3, Tppp3, is a specific marker of the differentiating tendon sheath and synovial joints. Dev. Dyn. 238, 685–692 (2009).
Harvey, T., Flamenco, S. & Fan, C. M. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell. Biol. 21, 1490–1503 (2019).
Xie, T. et al. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 22, 3625–3640 (2018).
Wei, J. et al. Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 64, 2734–2745 (2012).
Hwang, S. Y. et al. Direct targeting of β-catenin by a small molecule stimulates proteasomal degradation and suppresses oncogenic Wnt/β-catenin signaling. Cell Rep. 16, 28–36 (2016).
Meyer, M., Müller, A. K., Yang, J., Ŝulcová, J. & Werner, S. The role of chronic inflammation in cutaneous fibrosis: fibroblast growth factor receptor deficiency in keratinocytes as an example. J. Investig. Dermatol. Symp. Proc. 15, 48–52 (2011).
Ueha, S., Shand, F. H. & Matsushima, K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front. Immunol. 3, 71 (2012).
Wick, G. et al. The immunology of fibrosis: innate and adaptive responses. Trends Immunol. 31, 110–119 (2010).
MacDonald, B. T., Tamai, K. & He, X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 17, 9–26 (2009).
Piersma, B., Bank, R. A. & Boersema, M. Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front. Med. (Lausanne). 2, 59 (2015).
Burgy, O. & Königshoff, M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 68–69, 67–80 (2018).
Hamburg-Shields, E., DiNuoscio, G. J., Mullin, N. K., Lafyatis, R. & Atit, R. P. Sustained β-catenin activity in dermal fibroblasts promotes fibrosis by up-regulating expression of extracellular matrix protein-coding genes. J. Pathol. 235, 686–697 (2015).
Lam, A. P. et al. Nuclear β-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am. J. Respir. Cell Mol. Biol. 45, 915–922 (2011).
Henderson, W. R. et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. USA. 107, 14309–14314 (2010).
Cao, H. et al. Inhibition of Wnt/β-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci. Rep. 8, 13644 (2018).
Beyer, C. et al. β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann. Rheum. Dis. 71, 761–767 (2012).
Tokunaga, Y. et al. Selective inhibitor of Wnt/β-catenin/CBP signaling ameliorates hepatitis C virus-induced liver fibrosis in mouse model. Sci. Rep. 7, 325 (2017).
Chen, S., McLean, S., Carter, D. E. & Leask, A. The gene expression profile induced by Wnt 3a in NIH 3T3 fibroblasts. J. Cell Commun. Signal. 1, 175–183 (2007).
Działo, E. et al. WNT3a and WNT5a transported by exosomes activate WNT signaling pathways in human cardiac fibroblasts. Int. J. Mol. Sci. 20, 1436 (2019).
Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 217, e20190103 (2020).
Serini, G. & Gabbiana, G. Modulation of alpha-smooth muscle actin expression in fibroblasts by transforming growth factor-beta isoforms: an in vivo and in vitro study. Wound Repair Regen. 4, 278–287 (1996).
Akhmetshina, A. et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 3, 735 (2012).
Zhou, B. et al. Interactions between β-catenin and transforming growth factor-β signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J. Biol. Chem. 287, 7026–7038 (2012).
Hou, J. et al. M2 macrophages promote myofibroblast differentiation of LR-MSCs and are associated with pulmonary fibrogenesis. Cell Commun. Signal. 16, 89 (2018).
Wynn, T. A. & Vannella, K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 44, 450–462 (2016).
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 (2012).
Lodyga, M. et al. Cadherin-11-mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-β. Sci. Signal. 12, eaao3469 (2019).
Cereijo, R. et al. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab. 28, 750–763.e6 (2018).
Tomita, K. et al. CXCL10-mediates macrophage, but not other innate immune cells-associated inflammation in murine nonalcoholic steatohepatitis. Sci. Rep. 6, 28786 (2016).
Giri, J., Das, R., Nylen, E., Chinnadurai, R. & Galipeau, J. CCL2 and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ tissue macrophages to mitigate gut injury. Cell Rep. 30, 1923–1934.e4 (2020).
Lan, Q. et al. CCL26 participates in the PRL-3-induced promotion of colorectal cancer invasion by stimulating tumor-associated macrophage infiltration. Mol. Cancer Ther. 17, 276–289 (2018).
Wang, Y. et al. FGF-2 signaling in nasopharyngeal carcinoma modulates pericyte-macrophage crosstalk and metastasis. JCI Insight. 7, e157874 (2022).
Lu, J., Chatterjee, M., Schmid, H., Beck, S. & Gawaz, M. Chemokine CXCL14 acts as a potential genetic target for liver fibrosis. J. Inflamm. (Lond). 13, 1 (2016).
Wang, S. et al. Chemokine CXCL14 acts as a potential genetic target for liver fibrosis. Int. Immunopharmacol. 89, 107067 (2020).
Sturmlechner, I. et al. p21 produces a bioactive secretome that places stressed cells under immunosurveillance. Science. 374, eabb3420 (2021).
Jia, G. et al. CXCL14 is a candidate biomarker for Hedgehog signalling in idiopathic pulmonary fibrosis. Thorax. 72, 780–787 (2017).
Layton, T. B. et al. Cellular census of human fibrosis defines functionally distinct stromal cell types and states. Nat. Commun. 11, 2768 (2020).
Montgomery, E., Lee, J. H., Abraham, S. C. & Wu, T. T. Superficial fibromatoses are genetically distinct from deep fibromatoses. Mod. Pathol. 14, 695–701 (2001).
Tanegashima, K. et al. CXCL14 is a natural inhibitor of the CXCL12-CXCR4 signaling axis. FEBS Lett. 587, 1731–1735 (2013).
Wei, S. T. et al. Hypoxia-induced CXC chemokine ligand 14 expression drives protumorigenic effects through activation of insulin-like growth factor-1 receptor signaling in glioblastoma. Cancer Sci. 114, 174–186 (2023).
Tanegashima, K. et al. CXCL14 deficiency in mice attenuates obesity and inhibits feeding behavior in a novel environment. PLoS One. 5, e10321 (2010).
Nara, N. et al. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J. Biol. Chem. 282, 30794–30803 (2007).
Lai, X. et al. CXCL14 protects against polymicrobial sepsis by enhancing antibacterial functions of macrophages. Am. J. Respir. Cell Mol. Biol. 67, 589–601 (2022).
Komura, S. et al. Cell-type dependent enhancer binding of the EWS/ATF1 fusion gene in clear cell sarcomas. Nat. Commun. 10, 3999 (2019).
Hirata, A. et al. Dose-dependent roles for canonical Wnt signalling in de novo crypt formation and cell cycle properties of the colonic epithelium. Development. 140, 66–75 (2013).
Harada, N. et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO. J. 18, 5931–5942 (1999).
Akiyama, H. et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 18, 1072–1087 (2004).
Chytil, A., Magnuson, M. A., Wright, C. V. & Moses, H. L. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 32, 73–75 (2002).
Sueyoshi, T., Yamamoto, K. & Akiyama, H. Conditional deletion of Tgfbr2 in hypertrophic chondrocytes delays terminal chondrocyte differentiation. Matrix Biol. 31, 352–359 (2012).