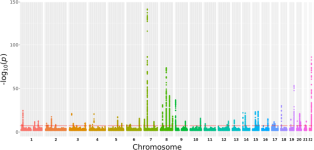
A genome-wide association meta-analysis implicates Hedgehog and Notch signaling in Dupuytren’s disease
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 (2012).
Kuo, R. Y. L., Ng, M., Prieto-Alhambra, D. & Furniss, D. Dupuytren’s disease predicts increased all-cause and cancer-specific mortality: analysis of a large cohort from the U.K. clinical practice research datalink. Plast. Reconstr. Surg. 145, 574e–582e (2020).
Lanting, R., Broekstra, D. C., Werker, P. M. N. & van den Heuvel, E. R. A systematic review and meta-analysis on the prevalence of Dupuytren disease in the general population of western countries. Plast. Reconstr. Surg. 133, 593–603 (2014).
Wilburn, J., McKenna, S. P., Perry-Hinsley, D. & Bayat, A. The impact of Dupuytren disease on patient activity and quality of life. J. Hand Surg. Am. 38, 1209–1214 (2013).
van Rijssen, A. L., ter Linden, H. & Werker, P. M. N. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast. Reconstr. Surg. 129, 469–477 (2012).
Layton, T. & Nanchahal, J. Recent advances in the understanding of Dupuytren’s disease [version 1; referees: 3 approved]. F1000 Res. 8, 1–8 (2019).
Alser, O. H., Kuo, R. Y. L. & Furniss, D. Nongenetic factors associated with Dupuytren’s disease: a systematic review. Plast. Reconstr. Surg. 74, 799–807 (2020).
Major, M. et al. Integrative analysis of Dupuytren’s disease identifies novel risk locus and reveals a shared genetic etiology with BMI. Genet. Epidemiol. 43, 629–645 (2019).
Majeed, M., Wiberg, A., Ng, M., Holmes, M. V. & Furniss, D. The relationship between body mass index and the risk of development of Dupuytren’s disease: a mendelian randomization study. J. Hand Surg. Eur. Vol. 46, 406–410 (2021).
Burkard, T. et al. The association of bariatric surgery and Dupuytren’s disease: a propensity score-matched cohort study. J. Hand Surg. Eur. Vol. 47, 288–295 (2022).
Dolmans, G. H. et al. Wnt signaling and Dupuytren’s disease. N. Engl. J. Med. 365, 307–317 (2011).
Ng, M. et al. A genome-wide association study of Dupuytren disease reveals 17 additional variants implicated in fibrosis. Am. J. Hum. Genet. 101, 417–427 (2017).
Ågren, R. et al. Major genetic risk factors for Dupuytren’ s disease are inherited from Neandertals. Mol. Biol. Evol. 40, 1–11 (2023).
Larsen, S. et al. Genetic and environmental influences in Dupuytren’s disease. J. Hand Surg. Eur. Vol. 40, 171–176 (2015).
Rentzsch, P., Witten, D., Cooper, G. M., Shendure, J. & Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nuc. Acids Res. 47, D886–D894 (2019).
Sato, M. Upregulation of the Wnt/β-catenin pathway induced by transforming growth factor-β in hypertrophic scars and keloids. Acta. Derm. Venereol. 86, 300–307 (2006).
Zhu, Z. et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J. Allergy Clin. Immunol. 145, 537–549 (2020). Feb.
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
Green, H., et al. A genome-wide association study identifies 5 loci associated with frozen shoulder and implicates diabetes as a causal risk factor. PLoS Genet. 10, 100–132 (2021).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in ~700 000 individuals of European ancestry. Hum. Mol. Genet. 27, 3641–3649 (2018).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016).
Layton, T. B. et al. A vasculature niche orchestrates stromal cell phenotype through PDGF signaling: Importance in human fibrotic disease. Proc. Natl Acad. Sci. USA 119, 1–11 (2022).
Ten Dam, E. J. P. M., van Beuge, M. M., Bank, R. A. & Werker, P. M. N. Further evidence of the involvement of the Wnt signaling pathway in Dupuytren’s disease. J. Cell Commun. Sign.10, 33–40 (2016).
Piersma, B. et al. YAP1 is a driver of myofibroblast differentiation in normal and diseased fibroblasts. Am. J. Pathol. 185, 3326–3337 (2015).
Piersma, B., Bank, R. A. & Boersema M. Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front. Med. 88, e00521 (2021).
Hu, L., Lin, X., Lu, H., Chen, B. & Bai, Y. An overview of hedgehog signaling in fibrosis. Mol. Pharm. 87, 174–182 (2015).
Omenetti, A. et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J. Clin. Investig. 118, 3331–3342 (2008).
Fitch, P. M., Howie, S. E. M. & Wallace, W. A. H. Oxidative damage and TGF-β differentially induce lung epithelial cell sonic hedgehog and tenascin-C expression: Implications for the regulation of lung remodelling in idiopathic interstitial lung disease. Int J. Exp. Pathol. 92, 8–17 (2011).
Shen, X., Peng, Y. & Li, H. The injury-related activation of hedgehog signaling pathway modulates the repair-associated inflammation in liver fibrosis. Front. Immunol. 8, 1450 (2017).
Dennler, S. et al. Induction of sonic hedgehog mediators by transforming growth factor-beta: smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 67, 6981–6986 (2007).
Berndt, A., Kosmehl, H., Katenkamp, D. & Tauchmann, V. Appearance of the myofibroblastic phenotype in Dupuytren’s disease is associated with a fibronectin, laminin, collagen type IV and tenascin extracellular matrix. Pathobiology 62, 55–58 (1994).
Fu, H. et al. Tenascin-C Is a major component of the fibrogenic niche in kidney fibrosis. J. Am. Soc. Nephrol. 28, 785–801 (2017).
Che, J. et al. Decreased expression of Dlg5 is associated with a poor prognosis and epithelial-mesenchymal transition in squamous cell lung cancer. J. Thorac. Dis. 13, 3115–3125 (2021).
Chong, Y. C., Mann, R. K., Zhao, C., Kato, M. & Beachy, P. A. Bifurcating action of smoothened in hedgehog signaling is mediated by Dlg5. Genes Dev. 29, 262–276 (2015).
Xie, J. Hedgehog signaling in prostate cancer. Future Oncol. 1, 331–338 (2005).
Hidalgo, M. & Maitra, A. The Hedgehog pathway and pancreatic cancer. N. Engl. J. Med. 361, 2094–2096 (2009).
Xie, J. Molecular biology of basal and squamous cell carcinomas. Adv. Exp. Med. Biol. 624, 241–251 (2008).
Bray, S. J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 17, 722–735 (2016).
Fan, J. et al. Targeting the Notch and TGF-β signaling pathways to prevent retinal fibrosis in vitro and in vivo. Theranostics 10, 7956–7973 (2020).
Zhang, K. et al. The liver-enriched lnc-LFAR1 promotes liver fibrosis by activating TGFβ and Notch pathways. Nat. Commun. 8, 144 (2017).
Edeling, M., Ragi, G., Huang, S., Pavenstädt, H. & Susztak, K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 12, 426–439 (2016).
Bielesz, B. et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Investig. 120, 4040–4054 (2010).
Hicks, C. et al. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat. Cell Biol. 2, 515–520 (2000).
Tian, J. et al. Loss of CHSY1, a secreted FRINGE enzyme, causes syndromic brachydactyly in humans via increased NOTCH signaling. Am. J. Hum. Genet. Inter. 87, 768–778 (2010).
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008).
Smith, S. P., Devaraj, V. S. & Bunker, T. D. The association between frozen shoulder and Dupuytren’s disease. J. Should. Elb. Surg. 10, 149–151 (2001).
Itoh, Y. et al. A common SNP risk variant MT1-MMP causative for Dupuytren’s disease has a specific defect in collagenolytic activity.pdf. Matrix Biol. 97, 20–39 (2021).
Hutchinson, J. W., Tierney, G. M., Parsons, S. L. & Davis, T. R. Dupuytren’s disease and frozen shoulder induced by treatment with a matrix metalloproteinase inhibitor. J. Bone Jt. Surg. Br. 80, 907–908 (1998).
van Beuge, M. M., ten Dam, E. J. P. M., Werker, P. M. N. & Bank, R. A. Wnt pathway in Dupuytren disease: connecting profibrotic signals. Transl. Res. 166, 762–771.e3 (2015).
Riesmeijer, S. A. et al. Polygenic risk associations with clinical characteristics and recurrence of Dupuytren’s disease. Plast Reconstr Surg. (2023). Online ahead of print.
Becker, K. et al. The importance of genetic susceptibility in Dupuytren’s disease. Clin. Genet. 87, 483–487 (2015).
Scholtens, S. et al. Cohort profile: lifeLines, a three-generation cohort study and biobank. Int J. Epidemiol. 44, 1172–1180 (2015).
Becker, K. et al. Meta-analysis of genome-wide association studies and network analysis-based integration with gene expression data identify new suggestive loci and unravel a Wnt-centric network associated with Dupuytren’s disease. PLoS One 11, 1–18 (2016).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Holle, R., Happich, M., Löwel, H. & Wichmann, H. E. KORA–a research platform for population based health research. Gesundheitswesen 67, S19–S25 (2005).
Nöthlings, U. & Krawczak, M. PopGen. A population-based biobank with prospective follow-up of a control group. Bundesgesundheitsbl. Gesundheitsforsch. Gesundheitssch. 55, 831–835 (2012).
Dolmans, G. H., de Bock, G. H. & Werker, P. M. Dupuytren diathesis and genetic risk. J. Hand Surg. 37, 2106–2111 (2012).
Laura, Fumagalli. & Nick, Buck. Understanding Society 14th edn, Vol. 6 (University of Essex Institute for Social and Economic Research, 2017).
Layton, T. B. et al. Cellular census of human fibrosis defines functionally distinct stromal cell types and states. Nat. Commun. 11, 1–11 (2020).
Purcell, S. et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Zheng, X. & Davis, J. W. SAIGEgds – an efficient statistical tool for large-scale PheWAS with mixed models. Bioinformatics 37, 728–730 (2021).
McCarthy, S. et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 48, 1279–1283 (2016).
Purcell, S. et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Marchini, J., Howie, B., Myers, S., McVean, G. & Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 39, 906–913 (2007).
Ani, A., van der Most, P., Snieder, H., Vaez, A. & Nolte, I. GWASinspector: comprehensive quality control of genome-wide association study results. Bioinformatics 37, 129–130 (2020).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Devlin, B. & Roeder, K. Genomic control for association studies. Biometrics 55, 997–1004 (1999).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1–10 (2017).
Vaez, A. et al. In silico post genome-wide association studies analysis of C-reactive protein Loci suggests an important role for interferons. Circ. Cardiovasc. Genet. 8, 487–497 (2015).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nuc. Acids Res. 38, e164 (2010).
Buniello, A. et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nuc. Acids Res. 47, D1005–D1012 (2019).
Kamali, Z., Keaton, J. M. & Javanmard, S. H. Large-scale multi-omics studies provide new insights into blood pressure regulation. Int J. Mol. Sci. 23, 7557 (2022).
Asefa, N. G. et al. Bioinformatic prioritization and functional annotation of GWAS-based candidate genes for primary open-angle Glaucoma. Genes Basel 13, 1055 (2022).
Benner, C. et al. FINEMAP: efficient variable selection using summary data from genome-wide association studies. Bioinformatics 32, 1493–1501 (2016).
Qi, T. et al. Identifying gene targets for brain-related traits using transcriptomic and methylomic data from blood. Nat. Commun. 9, 2282 (2018).
Slowikowski, K., Hu, X. & Raychaudhuri, S. SNPsea: an algorithm to identify cell types, tissues and pathways affected by risk loci. Bioinformatics 30, 2496–2497 (2014).
Galili, T., O’Callaghan, A., Sidi, J. & Sievert, C. heatmaply: an R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 34, 1600–1602 (2018).
Zheng Z. et al. Leveraging functional genomic annotations and genome coverage to improve polygenic prediction of complex traits within and between ancestries. bioRxiv (2022).
Lee, S. H., Goddard, M. E., Wray, N. R. & Visscher, P. M. A better coefficient of determination for genetic profile analysis. Genet Epidemiol. 36, 214–224 (2012).
Riesmeijer, S. A., Werker, P. M. N. & Nolte, I. M. Ethnic differences in prevalence of Dupuytren disease can partly be explained by known genetic risk variants. Eur. J. Hum. Genet. 27, 1876–1884 (2019).
Willer, C. et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet 45, 1274–1283 (2013).
Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 50, 1505–1513 (2018).
Chen, J. et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 53, 840–860 (2021).
Allen, R. J. et al. Genome-wide association study of susceptibility to idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 201, 564–574 (2020).
López-Isac, E. et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. Nat. Commun. 10, 4955 (2019).
Hindorff, L. A. et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl Acad. Sci. USA 106, 9362–9367 (2009).
Zheng, I. et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33, 272–279 (2017).
Morris, J. A. et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 51, 258–266 (2019).
Yengo, L. et al. A saturated map of common genetic variants associated with human height from 5.4 million individuals of diverse ancestries. bioRxiv (2022).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Wallace, C. A more accurate method for colocalisation analysis allowing for multiple causal variants. PLoS Genet. 17, e1009440 (2021).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, 1–19 (2015).
Pruim, R. J. et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337 (2010).